- Business Process Management (BPM)Document Management System (DMS)Electronic Quality Management System (QMS)Risk, Governance & Compliance (GRC)Low Code Rapid Application Development (LC)Business Continuity Management (BCM)Enterprise Architecture (EA)Business Process Management (BPM)Document Management System (DMS)
- Document Control Overview
- AI Content Creation & Improvement
- Policy & Procedure Management (SOP)
- AI Content Mining Parser
- Collaboration & Governance
- Data Migration & Integration
- Interfacing Offline App
 Electronic Quality Management System (QMS)
Electronic Quality Management System (QMS)- Quality Management System Overview
- Document Control & Records Management
- Audit & Accreditation Management
- Corrective & Preventative Action
- Quality Event (Non-conformity / Complaint/ Compliance)
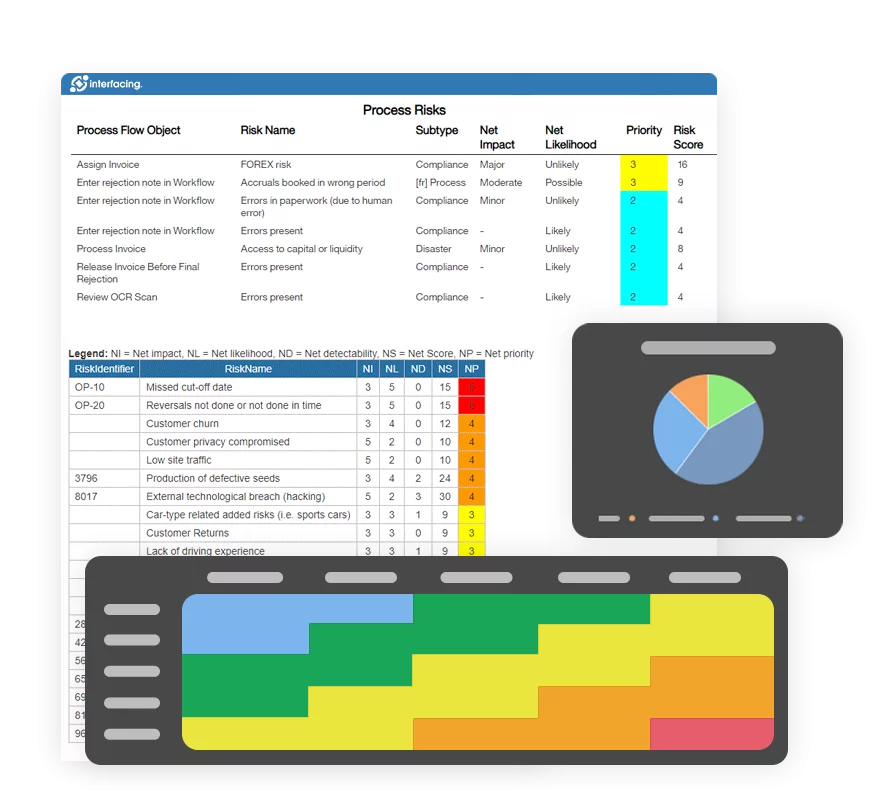
- Risk Management
- Incident Management
- Environmental Health & Safety
- Product & Supplier Management (SCAR)
- Training Management
- Control Management
- Action Items Management
- Management Review
- FMEA
- Pharmacovigilance
- Data Migration & Integration
 Risk, Governance & Compliance (GRC)
Risk, Governance & Compliance (GRC)- Risk, Governance & Compliance Overview
- Risk & Control Management
- Regulatory Compliance
- Collaboration & Governance
- Data Migration & Integration
- Interfacing Offline App
 Low Code Rapid Application Development (LC)
Low Code Rapid Application Development (LC)- Low Code Automation Platform Overview
- Electronic Web Form Design (eFORMS)
- Database Table Entity Designer
- System Integration Designer
- Design & Manage Tasks
- Design & Manage BPMS Apps
- Custom Rules/Guards/Actions
- Electronic Services
- User Homepage
- BAM (Business Activity Monitoring)
- Custom Dashboard Design
- Data Migration & Integration
 Business Continuity Management (BCM)
Business Continuity Management (BCM)- Business Continuity Management Overview
- Business Impact Analysis
- Disaster Recovery Simulation
- Action Item Management
- Mass Notification Management
- Asset Management
- Interfacing Offline App
 Enterprise Architecture (EA)
Enterprise Architecture (EA) - IndustriesRegulatory ComplianceUse CasesLearning CenterFramework & PracticesIndustries
- Healthcare
- Medical Device Technology
- Life Science, Pharmaceutical
- Aerospace & Defense
- Airlines and Aviation
- Media & Telecommunications
- Government and Military
- Technology
- Energy
- Logistics & Port Operations
- Banking & Capital Markets
- Retail & Consumer
- Consulting
- Education
- Engineering & Construction
- Manufacturing
- Financial Services
- Insurance
- Chemicals
Regulatory Compliance- Regulatory Compliance
- ISO
- ISO 9001 (guide)
- ISO 9001:2026 (preparation)
- ISO 17025
- ISO 27000
- ISO 27001
- ISO27002
- ISO 42001
- EU AI Act
- SOC 2 Type 1 & 2
- Sarbanes Oxley
- GxP
- GRC
- Basel
- Digital Signature
- GDPR
- IFRS
- NIST SP 800-53
 Use Cases
Use Cases- Quality Management System (QMS)
- Digital Transformation
- Continuous Improvement
- Governance, Risk & Compliance
- Knowledge Management
- System Deployment (ERP, CRM…)
 Learning CenterFramework & Practices
Learning CenterFramework & Practices - AboutCustomer SuccessPartners

Regulatory Compliance
Please Select contact form.
Is your Organization Compliant? Learn about the different regulatory compliances and how Interfacing can help you.

Is your Organization Compliant?
Learn about the different regulatory compliances and how Interfacing can help you.
Process Compliance – whether through government legislation, industry standards & regulations or a company’s own policies – is a prevalent business concern for all organizations. The cost of non-compliance can be both catastrophic and long-standing. Ignoring compliance can sometimes put your organization at great risk for not adhering to the law. The risk for reputation damages and the financial implications are important. Complying with regulation, legislation and policies can be a difficult task and managers need the right tools to ensure the organization is compliant.
A Solution Designed for the needs of Modern Industry
Companies implementing compliance have partnered with Interfacing to deploy workflow solutions involving management and automation of point-to-point business processes. This includes health sciences (including patient-related), manufacturing, administrative, financial and industry processes for example.
Interfacing’s process optimization & automation, SOP documentation digitalization and proactive regulatory vigilance solution, Enterprise Process Center© Suite has brought about significant time savings with accelerated approval & validation, improved visibility and operational improvements for customers including the following:
End-to-end alignment – Single Source of Truth
The EPC allows your organization to easily align departments, regulations, procedures, controls, within a single repository to enable quick and standardized continuous improvements. Our technology allows information fragmenting to build reusable data fragments.
Accelerated approvals and reviews
Interfacing EQMS solution delivers sustained time saving by reduced training, updating and re-training time. The integrated approval, review and endorsement workflow also helps streamline the approval of new version of the standard operating procedures (SOPs) through fragmented content review and approval cycles to aid decision-making.
Digital Signature and Multi-Factor Authentication
EPC meets the very stringent requirements associated with compliance for FSA QSR, ISO 9000, ISO 13845, GxP programs for example, and all SOPs and processes are parsed and documented, providing the audit trails meeting compliance with 21 CFR Part 11 Electronic Record and Electronic Signature (ERES) and a host of other regulatory requirements through RSA encryption, vault key story, and multi-factor authentication (MFA)
Improved Speed and Agility
Interfacing’s Enterprise Process Center© Suite is fully mobile and digital, providing your organization with the flexibility of quality documentation readily visible through a variety of means including phone, tablets, graphical and textual to boost employee agility and self-training.
Multi-language support, auto-translation and localized content
Interfacing’s Enterprise Process Center© Suite provides insightful ways to manage global content, whether by automated translation suggestions for all fragments of content, or the ability to collect variance for SOPs by country, region or product-type
Understanding compliance: ISO 9001, ISO 13485, FDA QSR and GxP
We understand that the requirements placed on medical device companies in terms of compliance are very high and that ISO 9001, ISO 13845, FDA QSR and GxP is an essential part of that program. By using our solutions, your company gains the accountability and consistency that will give you a cutting edge over your competition. Our tools ensure full visibility from end-to-end, all the way from the creation and amendment of a regulation to the approval and revision of the content through to the update and retraining of employees for standard operating procedures (SOPs). We see the full lifecycle management as moving parts of a complete ecosystem and that’s why are unique approach that combines regulatory requirements, documents, processes, work instructions, and governance.
ISO 9001
International standard that specifies requirements for a QMS. It is the most popular standard in the ISO 9000 series and the only standard in the series to which organizations can certify. First published in 1987 by the International Organization for Standardization (ISO). The current version of ISO 9001 was released in September 2015.
ISO 13485
In short, ISO 13485 is an internationally recognized standard that the following countries have adopted: Europe, Canada, Australia and other markets. Excluding Canada, the application of ISO 13485 is not a requirement but is the de facto standard in use today as a measurement of full QMS compliance set forth on medical device regulations.
US FDA QSR
The US FDA QSR (also known as 21 CFR Part 820) was introduced prior to ISO 13485. All medical device companies in the US are required to meet this standard for national distribution but must comply with both regulations in order to distribute devices internationally. US FDA QSR must also be met by international companies wanting to do business with US customers.
Nuanced QMS
Other countries will have their own criteria to meet nuanced QMS requirements. For example while both Brazil and Japan have their own requirements, they are both based on existing US FDA QSR and ISO 13485 standards. On a positive note, these standards achieve harmonized quality management requirements to meet US, Canadian, European and all other QMS standards in effect.
GxP Compliance
Our approach can help with all regulations and compliances related to pharma production and medical devices (GxP, CFR, GCP, GLP, GMP, HIPAA, ISO9001, medical devices regulations, SOX, BITS, CSA, FDA, FedRAMP, FIPS, FISMA, MHRA, NISP DoD, PCI DSS, SOC 2, U.S. SEC 17a-4 among others).
Manage Content
Manage the individual pieces of information, assign owners, and ensure governance through approval cycles, and change requests.
Digital Signature
We fully support digital signature to ensure that the audit trail of all content is secure, time-stamped, with accurate and complete copies of records available for inspection throughout the retention period.
Ensure Transparency
Full visibility to understand where records are used and their applicability. You can also maintain digital content with clear accountability, including roles and responsibilities.
Conduct Impact Analysis
Analyze your records for downstream impacts, and analyze the potential impacts on policies, SOPs, business units and related records.
Digital SOPs
Generate complete customizable output of processes and related records such as regulations in a ready-to-print and exportable Word format. You no longer need to manage SOP on paper! The digital SOP is in-sync all the time.
Approval and Governance Workflow
Integrated and embedded approval workflows to ensure strict control over the change of your records, including validation of changes, evaluation of impacts and highlighting changes.
Encourage Collaboration
By uniting goals and creating a common framework for your teams, they will be able to cooperate strategically, create change requests, and assign tasks to implementers.
ISO 27001 Certification at Interfacing
At Interfacing, we are dedicated to compliance and ensuring that our clients meet their regulatory requirements. As part of this commitment, we continuously strive to help organizations attain and maintain full compliance with international standards. Interfacing proudly holds ISO 27001 certification, reflecting our adherence to the highest standards of information security.
Cloud-Hosting Partnership with AWS
To strengthen our compliance initiatives, Interfacing has partnered with Amazon Web Services (AWS) for cloud-hosting. AWS’s commitment to compliance is well-established, with global data centers certified to SOC 1 Type II and ISO 27001 standards. This partnership ensures that our clients benefit from secure, reliable, and compliant cloud solutions.
AWS Compliance for Regulatory Standards
AWS’s compliance extends beyond ISO 27001 to support standards like ISO 18345, FDA QSR, and GxP. For more details on their comprehensive compliance program, please explore AWS's official compliance resources. This collaboration ensures that our cloud solutions meet the rigorous demands of global regulatory frameworks.
Why Choose Interfacing?
With over two decades of AI, Quality, Process, and Compliance software expertise, Interfacing continues to be a leader in the industry. To-date, it has served over 500+ world-class enterprises and management consulting firms from all industries and sectors. We continue to provide digital, cloud & AI solutions that enable organizations to enhance, control and streamline their processes while easing the burden of regulatory compliance and quality management programs.
To explore further or discuss how Interfacing can assist your organization, please complete the form below.

Documentation: Driving Transformation, Governance and Control
• Gain real-time, comprehensive insights into your operations.
• Improve governance, efficiency, and compliance.
• Ensure seamless alignment with regulatory standards.

eQMS: Automating Quality & Compliance Workflows & Reporting
• Simplify quality management with automated workflows and monitoring.
• Streamline CAPA, supplier audits, training and related workflows.
• Turn documentation into actionable insights for Quality 4.0

Low-Code Rapid Application Development: Accelerating Digital Transformation
• Build custom, scalable applications swiftly
• Reducing development time and cost
• Adapt faster and stay agile in the face of
evolving customer and business needs.
AI to Transform your Business!
The AI-powered tools are designed to streamline operations, enhance compliance, and drive sustainable growth. Check out how AI can:
• Respond to employee inquiries
• Transform videos into processes
• Assess regulatory impact & process improvements
• Generate forms, processes, risks, regulations, KPIs & more
• Parse regulatory standards into requirements

Request Free Demo
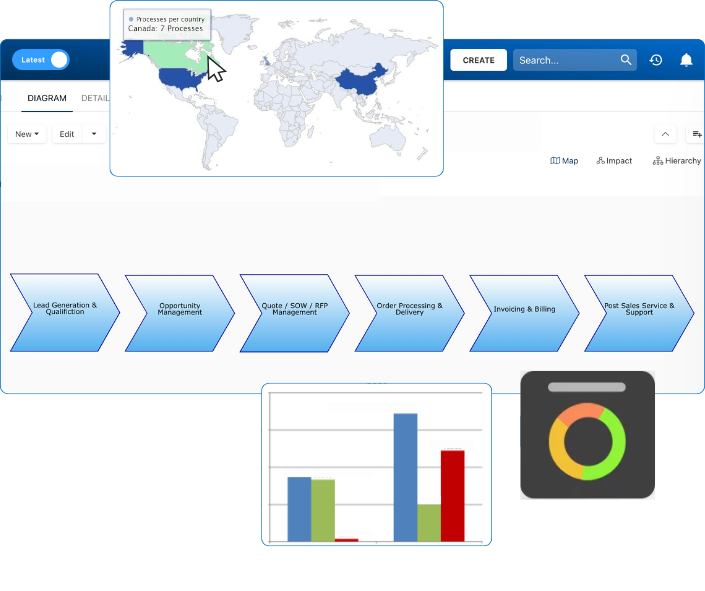
Document, analyze, improve, digitize and monitor your business processes, risks, regulatory requirements and performance indicators within Interfacing’s Digital Twin integrated management system the Enterprise Process Center®!
Trusted by Customers Worldwide!
More than 400+ world-class enterprises and management consulting firms