Adapting to Work in a New Normal – eQMS can help but dQMS is the Future!

eQMS – Electronic Quality Management System
In a new normal of having to work offsite, whether temporarily or permanently, planned, or in response to a crisis, it is a near impossibility to be in a position to successfully “bring the paperwork with you” at all times. Technology (i.e. eQMS) is quickly changing the way organizations manage their document creation, delivery, and storage processes. Even in today’s world, we cannot merely rely on actual paper productivity to remain ahead of the competition, not to mention regulation and compliance. Time, money, productivity, and governance are all affected by any quality management system (QMS) in place. For some organizations, locating, vetting, and implementing an Electronic Quality Management System (eQMS) is a critical and sometimes intimidating prospect for them to complete. It involves a significant change in business processes, and that can take months to finish.
Growing companies, and especially those looking to expand, will need to embrace technology processes. The critical factor is that choosing a specific technology process will determine its success or failure immediately – choose your process wisely. On the upside, though, to have a system in place that keeps records up to date automatically, has best practices built-into the workflow, provides easy and secure access to documents, aligns with all regulatory compliance, and makes validation effortless would be near perfection.
All organizations need to do an internal review. Even a basic one to determine how documents are shared, how updates and changes are handled or tracked, and how validation and compliance challenges are met would quickly identify potential issues. The goal here would be to streamline procedures and processes, improve risk management, and increase document and process visibility.
Paper Documents – A Challenge in Today’s Business
Let’s first address the fact that no company will go 100% paperless. An eQMS is there to manage all documents, but there will be time, contracts, legal, financial or medical documents that will need to be kept in hardcopy. Depending on whether your company employs digital signatures, the hardcopy will still be archived electronically and securely stored.
For the rest of the business processes, even meetings can become a challenge with paperwork. Imagine most of your offsite and hybrid meetings (both off and onsite). Without eQMS, the preparation collection and presentation of data is cumbersome as each attendee offsite will need to be provided with the same documentation. Further, nobody in the meeting will be receiving real-time data as the documentation in front of each individual is a snapshot of the time the information was recorded. Up to date document distribution can quickly dampen teamwork and collaboration for these purposes. The matter of editing critical documents to ensure they are accurate and up to date is a challenge in a non-eQMS environment. While basic solutions can store electronically (Google Drive, Dropbox, etc.), when it comes to version control, validation and regulatory compliance (for example medical industry FDA 21 CFR Part 11, none of those systems will offer the high-level tracking that an eQMS will provide.
The Accuracy of Audits
Every quality run organization needs to be audit-ready at all times. Without an eQMS, this can be a painstakingly long process to prepare for that is next to impossible to comply with. Paper-based documentation will get lost, and at the most inopportune time, this can be an embarrassment or the start of a search for more documentation that was previously not a part of the audit.
Lack of document control can cost significant time – dipping into the precious commodity that leaders must be conscious of daily. Even with simply emailing a document, this can create major problems.
Imagine sending one document via email to one or more stakeholders for signature. It has to go through many inboxes (stakeholder or their assistant, etc.) before it is sent to the next one. One missing signature means the process is delayed. Ad-hoc filing systems rarely receive validation and will create a burden come audit time.
With eQMS systems, you will generate the documentation for signature and notify those who must authorize/digitally sign-off. Each will click to approve or sign the document that sits securely in a single space in the repository.
Every quality run organization needs to be audit-ready at all times
Regulations and Compliance

It is a fact that regulatory changes will come with a specific frequency over the course of a year. This is a positive step towards keeping organizations ahead of the curve on many major compliance factors. What is a concern is whether an organization that is in a highly regulated discipline or industry can successfully keep up with the changes without an eQMS in place.
It takes time and effort to remain compliant, especially in federally regulated financial, medical, or other governmental bodies.
With a planned implementation, an organization can embrace regulatory changes very easily with an eQMS. Version control, auditing, time saved in securing the documentation to submit to the authorities are only a few examples of an eQMS’s strength in maintaining compliance.
Electronic Documents is Not Enough, The Future is Digital (DQMS)!
Converting paper documents to electronic format and storing them in a folder simply copies the same manual process that created the original issues in the first place regarding document control and continuity. In a short period of time, these documents will go out of date and be manually updated with no record of change. Multiple versions will exist that may pose a major liability to the organization if accidentally delivered to customers or clients.
Digital QMS (dQMS) provides consistency, continuity, and data management aligned with industry 4.0 and all of its benefits: usability, analysis, visibility, etc., all integrated within an AI (artificial intelligence) platform.

Digital QMS – Manage Data not Documents!
Paper-based QMS’s are an antiquated system that cannot meet today’s technological requirements from other systems used by partners, vendors, auditors, governmental agencies, etc. Recognizing the need to organize and secure your documentation for onsite and remote workers is a great first step in the right direction.
A dQMS will bring about standardization across all functions and regulatory silos, providing employees with a single, credible source of truth in any specific documentation. For example, any external change to a regulatory document would automatically flag the system and trigger revisions to quality documentation. This will keep critical documentation in its most current version right at the source without any other form of that documentation becoming available and posing a potential liability to the organization. Moving to a Digital Quality Management System means re-imagining how your employees will interact with SOPs, corporate, and legal documentation to name a few. What was once a tedious process to maintain document control, now becomes an intuitive process through a seamless digital interface, in addition to significant manual update time savings.
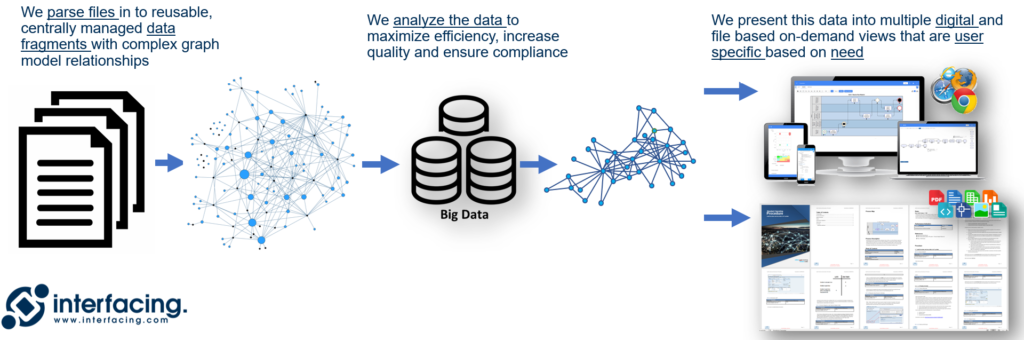
Transparent communication, continuous improvement, and agility are all benefits to your organization by integrating a dQMS data-driven approach, setting the stage for industry 4.0 digital transformation. By managing content as data fragments, our platform can process that data within AI (artificial intelligence) models to not only ensure compliance, but to be proactive vs.reactive to market changes and allowing you to pivot faster than your competitors!

Request Free Demo
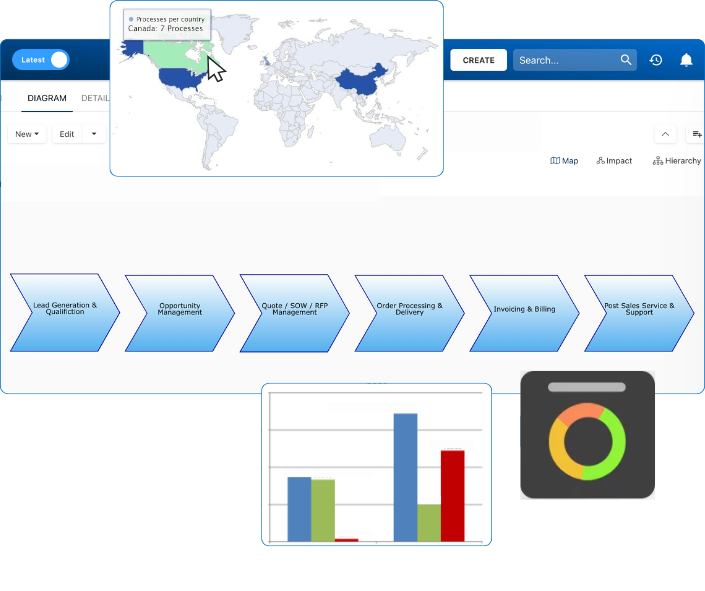
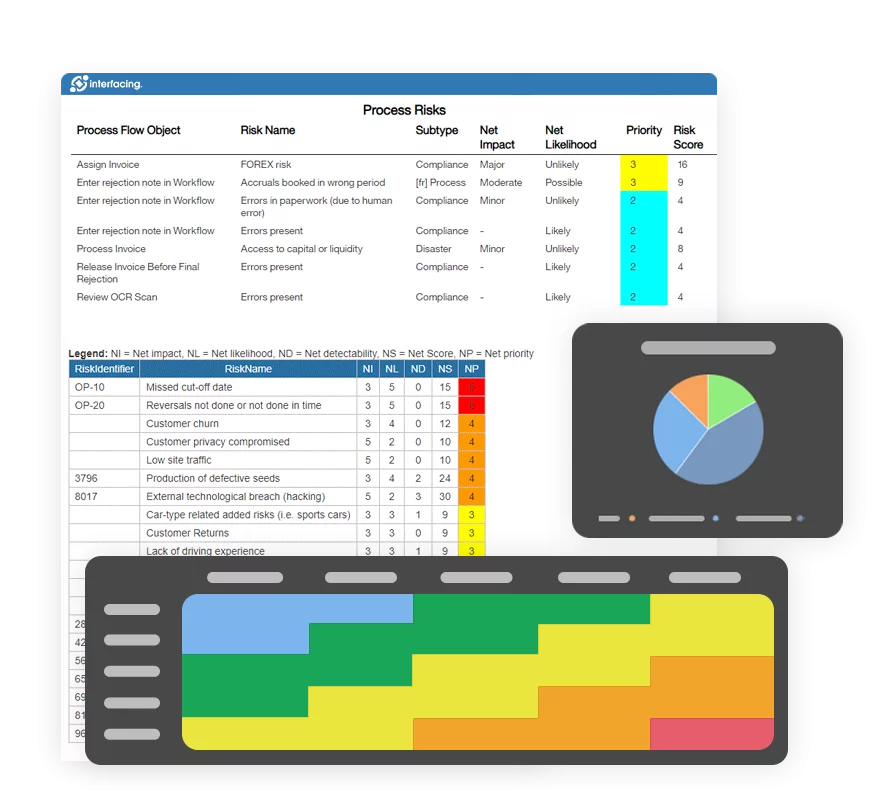
Document, analyze, improve, digitize and monitor your business processes, risks, regulatory requirements and performance indicators within Interfacing’s Digital Twin integrated management system the Enterprise Process Center®!