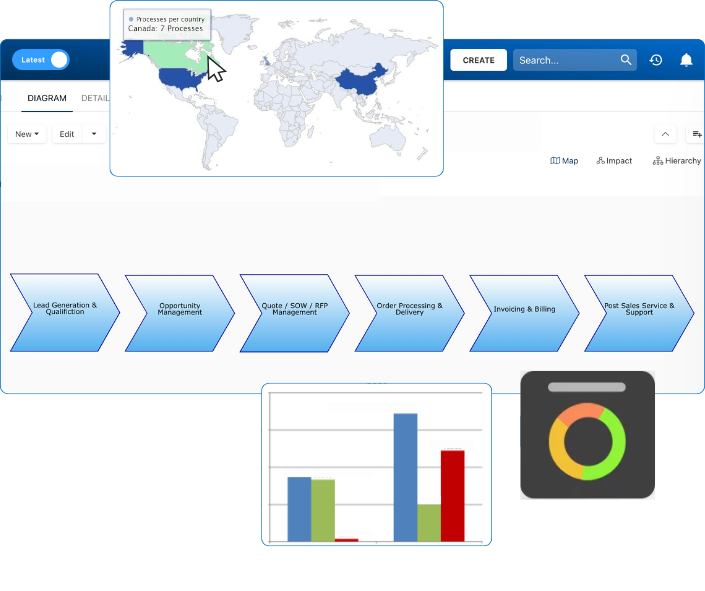
- Business Process Management (BPM)Document Management System (DMS)Electronic Quality Management System (QMS)Risk, Governance & Compliance (GRC)Low Code Rapid Application Development (LC)Business Continuity Management (BCM)Enterprise Architecture (EA)Business Process Management (BPM)Document Management System (DMS)
- Document Control Overview
- AI Content Creation & Improvement
- Policy & Procedure Management (SOP)
- AI Content Mining Parser
- Collaboration & Governance
- Data Migration & Integration
- Interfacing Offline App
 Electronic Quality Management System (QMS)
Electronic Quality Management System (QMS)- Quality Management System Overview
- Document Control & Records Management
- Audit & Accreditation Management
- Corrective & Preventative Action
- Quality Event (Non-conformity / Complaint/ Compliance)
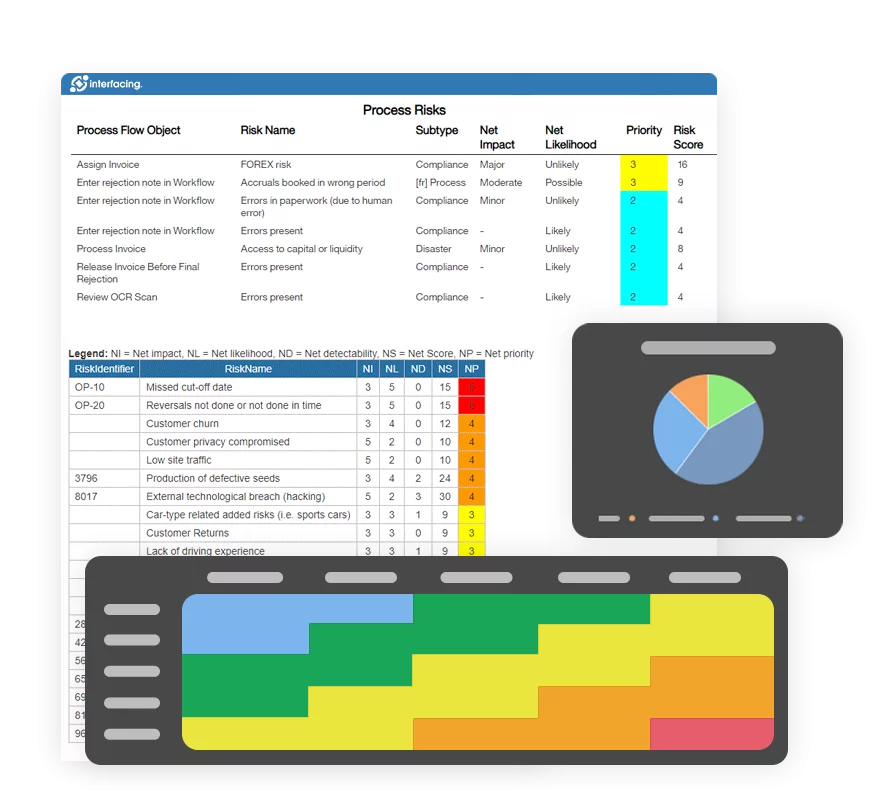
- Risk Management
- Incident Management
- Environmental Health & Safety
- Product & Supplier Management (SCAR)
- Training Management
- Control Management
- Action Items Management
- Management Review
- FMEA
- Pharmacovigilance
- Data Migration & Integration
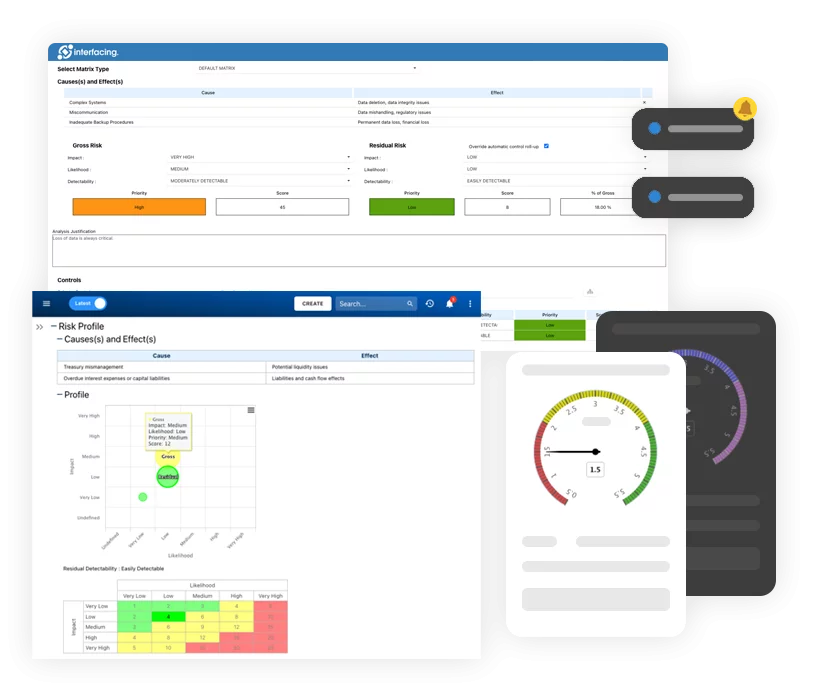
 Risk, Governance & Compliance (GRC)
Risk, Governance & Compliance (GRC)- Risk, Governance & Compliance Overview
- Risk & Control Management
- Regulatory Compliance
- Collaboration & Governance
- Data Migration & Integration
- Interfacing Offline App
 Low Code Rapid Application Development (LC)
Low Code Rapid Application Development (LC)- Low Code Automation Platform Overview
- Electronic Web Form Design (eFORMS)
- Database Table Entity Designer
- System Integration Designer
- Design & Manage Tasks
- Design & Manage BPMS Apps
- Custom Rules/Guards/Actions
- Electronic Services
- User Homepage
- BAM (Business Activity Monitoring)
- Custom Dashboard Design
- Data Migration & Integration
 Business Continuity Management (BCM)
Business Continuity Management (BCM)- Business Continuity Management Overview
- Business Impact Analysis
- Disaster Recovery Simulation
- Action Item Management
- Mass Notification Management
- Asset Management
- Interfacing Offline App
 Enterprise Architecture (EA)
Enterprise Architecture (EA) - IndustriesRegulatory ComplianceUse CasesLearning CenterFramework & PracticesIndustries
- Healthcare
- Medical Device Technology
- Life Science, Pharmaceutical
- Aerospace & Defense
- Airlines and Aviation
- Media & Telecommunications
- Government and Military
- Technology
- Energy
- Logistics & Port Operations
- Banking & Capital Markets
- Retail & Consumer
- Consulting
- Education
- Engineering & Construction
- Manufacturing
- Financial Services
- Insurance
- Chemicals
Regulatory Compliance- Regulatory Compliance
- ISO
- ISO 9001 (guide)
- ISO 9001:2026 (preparation)
- ISO 17025
- ISO 27000
- ISO 27001
- ISO27002
- ISO 42001
- EU AI Act
- SOC 2 Type 1 & 2
- Sarbanes Oxley
- GxP
- GRC
- Basel
- Digital Signature
- GDPR
- IFRS
- NIST SP 800-53
 Use Cases
Use Cases- Quality Management System (QMS)
- Digital Transformation
- Continuous Improvement
- Governance, Risk & Compliance
- Knowledge Management
- System Deployment (ERP, CRM…)
 Learning CenterFramework & Practices
Learning CenterFramework & Practices - AboutCustomer SuccessPartners

Life Science & Pharmaceutical
Please Select contact form.
Learn how Interfacing can help make your GxP digital quality program better

Digital Quality Management Software (QMS) for Medical Technology & Pharmaceuticals
Every aspect of the medical technology and pharmaceutical manufacturing process must be tightly controlled and monitored to comply with ISO 13485, ISO 9001, and FDA QSR (Quality System Regulation) requirements. To maintain operational integrity and ensure product quality, adherence to global standards like GMP (Good Manufacturing Practices) and GxP compliance frameworks is essential.
Interfacing’s Digital QMS combines AI-driven intelligence with process-centric control to deliver real-time oversight, automated traceability, and predictive compliance management. From product development through supply chain and shipping, our AI-enhanced solutions help organizations transition from manual, fragmented systems to streamlined digital operations—boosting both compliance confidence and business performance.
A Solution Designed for the needs of the
Medical Technology Industry
Interfacing’s process optimization & automation, SOP documentation digitalization and proactive regulatory vigilance solution, the Enterprise Process Center© Suite has brought about significant time savings with accelerated approval & validation, improved visibility and operational improvements for customers including the following:
End-to-end alignment
End-to-end alignment
The EPC allows life science organizations to easily align departments, regulations, procedures, controls, within a single repository to enable quick and standardized continuous improvements. Our technology allows information fragmenting to build reusable data fragments.
Accelerated approvals and validation
Accelerated approvals and validation
Interfacing QMS solution delivers sustained time saving by reduced training, updating and re-training time. The integrated approval, review and endorsement workflow also helps streamline the validation of new version of the standard operating procedures (SOPs) through fragmented content review and approval cycles to aid decision-making.
Digital Signature and Multi-Factor Authentication
Digital Signature and Multi-Factor Authentication
EPC meets the very stringent requirements associated with compliance for FSA QSR, ISO 13845, GxP programs, and all SOPs and processes are parsed and documented, providing the audit trails meeting compliance with 21 CFR Part 11 Electronic Record and Electronic Signature (ERES) and a host of other regulatory requirements through RSA encryption, vault key story, and multi-factor authentication (MFA)
Improved Speed and Agility
Improved Speed and Agility
Interfacing’s Enterprise Process Center© Suite is fully mobile and digital, providing life science organization with the flexibility of quality documentation readily visible through a variety of means including phone, tablets, graphical and textual to boost employee agility and self-training.
Multi-language support
Multi-language support
Interfacing’s Enterprise Process Center© Suite provides insightful ways to manage global content, whether by automated translation suggestions for all fragments of content, or the ability to collect variance for SOPs by country, region or product-type
Good Pharmaceutical Manufacturing Practices
We understand that the compliance requirements for pharmaceutical companies are among the most stringent in the world—and GxP is a foundational pillar. Interfacing’s digital solutions go beyond traditional compliance by incorporating artificial intelligence to ensure full visibility and traceability across the pharmaceutical value chain. From document creation and versioning to automated impact analysis and real-time audit readiness, our AI-powered EPC Suite helps reduce human error, accelerate validation cycles, and ensure data integrity. With an ecosystem approach that unifies people, processes, systems, and documents, we provide the agility and control needed to maintain compliance with evolving regulations such as ISO 13485, FDA QSR, and Annex 11.
GxP Compliance
Our approach can help with all regulations and compliances related to pharma production and medical devices (GxP, CFR, GCP, GLP, GMP, HIPAA, ISO9001, medical devices regulations, SOX, BITS, CSA, FDA, FedRAMP, FIPS, FISMA, MHRA, NISP DoD, PCI DSS, SOC 2, U.S. SEC 17a-4 among others).
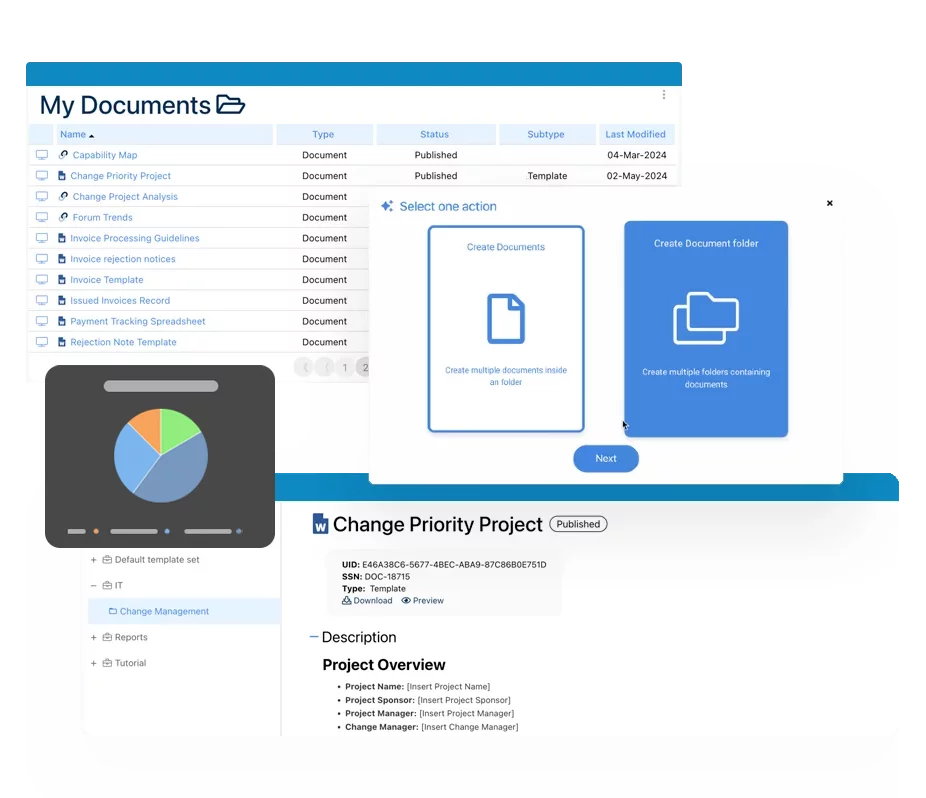
Manage Content
Manage the individual pieces of information, assign owners, and ensure governance through approval cycles, and change requests.
Digital Signature
We fully support digital signature to ensure that the audit trail of all content is secure, time-stamped, with accurate and complete copies of records available for inspection throughout the retention period.
Ensure Transparency
Full visibility to understand where records are used and their applicability. You can also maintain digital content with clear accountability, including roles and responsibilities.
Conduct Impact Analysis
Analyze your records for downstream impacts, and analyze the potential impacts on policies, SOPs, business units and related records.
Digital SOPs
Generate complete customizable output of processes and related records such as regulations in a ready-to-print and exportable Word format. You no longer need to manage SOP on paper! The digital SOP is in-sync all the time.
Approval and Governance Workflow
Integrated and embedded approval workflows to ensure strict control over the change of your records, including validation of changes, evaluation of impacts and highlighting changes.
Encourage Collaboration
By uniting goals and creating a common framework for your teams, they will be able to cooperate strategically, create change requests, and assign tasks to implementers.
Commitment to Compliance
As part of our ongoing commitment to compliance and ensuring that our clients meet their regulatory requirements, we are always on the lookout for ways to help our clients attain and maintain full compliance.
ISO 27001 Certification
Interfacing is ISO 27001 certified, demonstrating our commitment to top-tier information security and client data protection. We provide a secure, compliant environment clients can trust.
Partnership with AWS
We're partnering with Amazon Web Services (AWS) for cloud hosting due to their proven compliance standards. AWS’s global data centers meet SOC 1 Type II and ISO 27001. For details on their compliance with ISO 18345, FDA QSR, and GxP, see their compliance program.
AI-Driven Compliance Automation
Harness artificial intelligence to streamline regulatory compliance across the pharmaceutical lifecycle. Interfacing’s EPC Suite automatically maps current-state processes, monitors regulatory updates (FDA QSR, ISO 13485, EU Annex 11), and suggests changes to SOPs and quality documents. Embedded smart prompts reduce manual oversight and help ensure audit readiness at all times.
AI-Enhanced Digital QMS for Medical Technology & Pharmaceuticals
The complexity of pharmaceutical and MedTech manufacturing requires precision, documentation traceability, and strict regulatory compliance. Interfacing’s Digital Quality Management Software, integrated with artificial intelligence, empowers organizations to meet these demands with agility and control.
AI supports QMS functions by automatically classifying, tagging, and indexing regulatory documents and SOPs, ensuring compliance with GxP, ISO 13485, and FDA 21 CFR Part 11. With AI-powered semantic search and metadata-driven governance, quality managers can instantly retrieve validated information and detect inconsistencies across product life cycles. Predictive insights generated through process mining tools help identify deviation trends and recommend preventive actions, reducing CAPA fatigue and supporting continuous improvement.
By integrating AI into quality oversight, pharmaceutical companies can ensure accurate batch records, faster audit preparation, and proactive risk management across manufacturing and clinical workflows.




Request Free Demo
Document, analyze, improve, digitize and monitor your business processes, risks, regulatory requirements and performance indicators within Interfacing’s Digital Twin integrated management system the Enterprise Process Center®!